Abstract
Background: Recent studies have identified clinical and genomic factors contributing to worse clinical outcomes in patients with multiple myeloma (MM). Clonal hematopoiesis (CH) reflects the presence of somatic driver mutations in the blood or marrow of otherwise asymptomatic individuals. Using a variant allele frequency (VAF) cutoff of 2%, we recently reported CH in 21.6% of MM patients at the time of autologous stem cell transplant (ASCT) and found it was associated with shorter overall survival (OS) and progression-free survival (PFS) in those who did not receive maintenance therapy with an immunomodulatory drug (IMiD). However, this finding was based on a single tertiary center and only included MM patients who received ASCT.
Methods: We studied a larger cohort of 986 newly diagnosed MM cases. Whole-exome sequencing (WES) data of peripheral blood and bone marrow samples of 986 MM patients (523 transplanted and 463 non-transplanted) from the Multiple Myeloma Research Foundation (MMRF) Clinical Outcomes in MM to Personal Assessment of Genetic Profile (CoMMpass, NCT0145429) study were analyzed. Both peripheral blood and tumor samples were analyzed to filter out myeloma mutations that could be contaminating the peripheral blood. Given the lower depth of coverage compared to prior targeted sequencing studies, small clones with a VAF below 2% were not detected. Altogether, the WES samples had a total depth of coverage of 117.68X. All data were analyzed using R version 3.5.0 (R Core Team).
Results: Among the total cohort, 113 CH mutations were detected in 101/986 (10.24%) patients. CH was detected in 42/523 (8.03%) transplanted patients, compared to 59/463 (12.74%) non-transplanted patients. The most commonly mutated genes were DNMT3A, TET2, ASXL1, PPM1D, and TP53. The median age of the cohort was 63 years (range: 27 - 93), 60% were male, and median follow-up was 3.9 years (95% CI: 3.7 - 4.0).
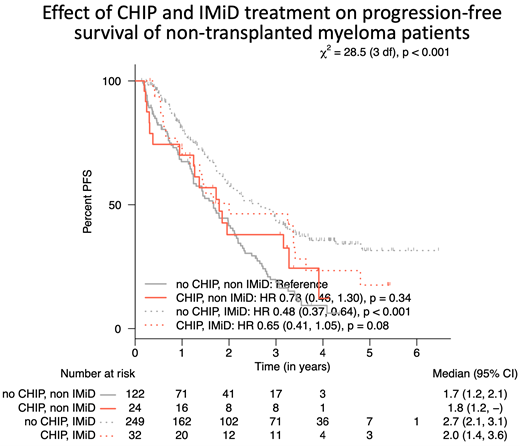
The presence of CH was associated with age (69 vs. 62 years, P < 0.001). As expected, the median age of transplanted patients was lower (60 vs. 67 years) than in the non-transplanted group, which likely explains the higher prevalence of CH detected in the non-transplanted group. CH was associated with recurrent bacterial infections (P = 0.01) and increased cardiovascular disease (P = 0.006), but not with cerebrovascular disease (P = 0.74) or coagulopathies (P = 0.65). There was a trend towards worse PFS in non-ASCT patients with CH who were not treated with IMiDs (1.8 years) compared to non-CH IMiD-treated patients (2.7 years) (P < 0.001). A CH effect on PFS was not detected in ASCT patients. OS was not different in those with or without CH in both ASCT and non-ASCT groups.
8 (0.8%) patients developed a second hematologic malignancy. CH at the time of MM diagnosis was not associated with an increased risk of developing a second hematologic malignancy (P = 0.58). To determine whether CH clones emerged or evolved during treatment, we examined serial samples from 52 patients (36 ASCT patients and 16 non-transplanted patients) with sequential samples. The median time between the first and second time point was 3.1 years (range: 1.0 - 5.4 years). At the first time point, only 3/52 (5.8%) patients had CH, but that number increased to 13/52 (25.0%) at the second time point. Five out of the 13 (38%) were non-transplanted patients. All but 1 patient were exposed to IMiDs. The most common emerging mutated gene was DNMT3A, found in 7 patient samples at the second time point, compared to 2 patients at the first time point.
Conclusion: Using WES in a large cohort of newly diagnosed MM patients, we detected CH in 10.2% (VAF ≥ 2%) of patients. CH and non-IMiD treatment confers a shorter PFS in non-transplanted MM patients. However, throughout IMiD-based treatment, MM patients tend to acquire and/or expand previously undetected CH clones, particularly DNMT3A. The clinical significance of this clonal expansion during therapy is yet to be elucidated, and for now, this observation does not yet change clinical management.
Steensma: Novartis: Current Employment. Ebert: Deerfield: Research Funding; GRAIL: Consultancy; Exo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Skyhawk Therapeutics: Membership on an entity's Board of Directors or advisory committees. Soiffer: NMPD - Be the Match, USA: Membership on an entity's Board of Directors or advisory committees; Gilead, USA: Other: Career Development Award Committee; Rheos Therapeutics, USA: Consultancy; Kiadis, Netherlands: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics, USA: Other: Data Safety Monitoring Board; Precision Biosciences, USA: Consultancy; Jazz Pharmaceuticals, USA: Consultancy; Jasper: Consultancy; Takeda: Consultancy. Sperling: Adaptive: Consultancy. Getz: Scorpion Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees; IBM, Pharmacyclics: Research Funding. Ghobrial: AbbVie, Adaptive, Aptitude Health, BMS, Cellectar, Curio Science, Genetch, Janssen, Janssen Central American and Caribbean, Karyopharm, Medscape, Oncopeptides, Sanofi, Takeda, The Binding Site, GNS, GSK: Consultancy.